 Index
Index

Lipids consist of numerous fatlike chemical compounds that are insoluble in water but soluble in organic solvents. Lipid compounds include monoglycerides, diglycerides, triglycerides, phosphatides, cerebrosides, sterols, terpenes, fatty alcohols, and fatty acids. Dietary fats supply energy, carry fat-soluble vitamins (A, D, E, K), and are a source of antioxidants and bioactive compounds. Fats are also incorporated as structural components of the brain and cell membranes.

| Chemical Names and Descriptions of some Common Fatty Acids | ||||
| Common Name |
Carbon Atoms |
Double Bonds |
Scientific Name | Sources |
| Butyric acid | 4 | 0 | butanoic acid | butterfat |
| Caproic Acid | 6 | 0 | hexanoic acid | butterfat |
| Caprylic Acid | 8 | 0 | octanoic acid | coconut oil |
| Capric Acid | 10 | 0 | decanoic acid | coconut oil |
| Lauric Acid | 12 | 0 | dodecanoic acid | coconut oil |
| Myristic Acid | 14 | 0 | tetradecanoic acid | palm kernel oil |
| Palmitic Acid | 16 | 0 | hexadecanoic acid | palm oil |
| Palmitoleic Acid | 16 | 1 | 9-hexadecenoic acid | animal fats |
| Stearic Acid | 18 | 0 | octadecanoic acid | animal fats |
| Oleic Acid | 18 | 1 | 9-octadecenoic acid | olive oil |
| Ricinoleic acid | 18 | 1 | 12-hydroxy-9-octadecenoic acid | castor oil |
| Vaccenic Acid | 18 | 1 | 11-octadecenoic acid | butterfat |
| Linoleic Acid | 18 | 2 | 9,12-octadecadienoic acid | grape seed oil |
|
Alpha-Linolenic Acid (ALA) |
18 | 3 | 9,12,15-octadecatrienoic acid |
flaxseed (linseed) oil |
|
Gamma-Linolenic Acid (GLA) |
18 | 3 | 6,9,12-octadecatrienoic acid | borage oil |
| Arachidic Acid | 20 | 0 | eicosanoic acid |
peanut oil, fish oil |
| Gadoleic Acid | 20 | 1 | 9-eicosenoic acid | fish oil |
| Arachidonic Acid (AA) | 20 | 4 | 5,8,11,14-eicosatetraenoic acid | liver fats |
| EPA | 20 | 5 | 5,8,11,14,17-eicosapentaenoic acid | fish oil |
| Behenic acid | 22 | 0 | docosanoic acid | rapeseed oil |
| Erucic acid | 22 | 1 | 13-docosenoic acid | rapeseed oil |
| DHA | 22 | 6 |
4,7,10,13,16,19-docosahexaenoic acid |
fish oil |
| Lignoceric acid | 24 | 0 | tetracosanoic acid |
small amounts in most fats |
Fatty acids consist of the elements carbon (C), hydrogen (H) and oxygen (O) arranged as a carbon chain skeleton with a carboxyl group (-COOH) at one end. Saturated fatty acids (SFAs) have all the hydrogen that the carbon atoms can hold, and therefore, have no double bonds between the carbons. Monounsaturated fatty acids (MUFAs) have only one double bond. Polyunsaturated fatty acids (PUFAs) have more than one double bond.
 Butyric Acid
Butyric Acid
Butyric acid (butanoic acid) is one of the saturated short-chain fatty acids responsible for the characteristic flavor of butter. This image is a detailed structural formula explicitly showing four bonds for every carbon atom and can also be represented as the equivalent line formulas:
The numbers at the beginning of the scientific names indicate the locations of the double bonds. By convention, the carbon of the carboxyl group is carbon number one. Greek numeric prefixes such as di, tri, tetra, penta, hexa, etc., are used as multipliers and to describe the length of carbon chains containing more than four atoms. Thus, "9,12-octadecadienoic acid" indicates that there is an 18-carbon chain (octa deca) with two double bonds (di en) located at carbons 9 and 12, with carbon 1 constituting a carboxyl group (oic acid). The structural formula corresponds to:
Fatty acids are frequently represented by a notation such as C18:2 that indicates that the fatty acid consists of an 18-carbon chain and 2 double bonds. Although this could refer to any of several possible fatty acid isomers with this chemical composition, it implies the naturally-occurring fatty acid with these characteristics, i.e., linoleic acid. Double bonds are said to be "conjugated" when they are separated from each other by one single bond, e.g., (-CH=CH-CH=CH-). The term "conjugated linoleic acid" (CLA) refers to several C18:2 linoleic acid variants such as 9,11-CLA and 10,12-CLA which correspond to 9,11-octadecadienoic acid and 10,12-octadecadienoic acid. The principal dietary isomer of CLA is cis-9,trans-11 CLA, also known as rumenic acid. CLA is found naturally in meats, eggs, cheese, milk and yogurt.
Double bonds bind carbon atoms tightly and prevent rotation of the carbon atoms along the bond axis. This gives rise to configurational isomers which are arrangements of atoms that can only be changed by breaking the bonds.
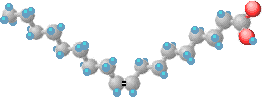

These three-dimensional molecular projections show the Cis and Trans configurational isomers of 9-octadecenoic acid with the hydrogen atoms shown in blue. The Latin prefixes Cis and Trans describe the orientation of the hydrogen atoms with respect to the double bond. Cis means "on the same side" and Trans means "across" or "on the other side". Naturally occurring fatty acids generally have the Cis configuration. The natural form of 9-octadecenoic acid (oleic acid) found in olive oil has a "V" shape due to the Cis configuration at position 9. The Trans configuration (elaidic acid) looks more like a straight line.
|
|
|
| Cis Configuration | Trans Configuration |
Omega-3 (ω3) and omega-6 (ω6) fatty acids are unsaturated "Essential Fatty Acids" (EFAs) that need to be included in the diet because the human metabolism cannot create them from other fatty acids. Since these fatty acids are polyunsaturated, the terms n-3 PUFAs and n-6 PUFAs are applied to omega-3 and omega-6 fatty acids, respectively. These fatty acids use the Greek alphabet (α,β,γ,...,ω) to identify the location of the double bonds. The "alpha" carbon is the carbon closest to the carboxyl group (carbon number 2), and the "omega" is the last carbon of the chain because omega is the last letter of the Greek alphabet. Linoleic acid is an omega-6 fatty acid because it has a double bond six carbons away from the "omega" carbon. Linoleic acid plays an important role in lowering cholesterol levels. Alpha-linolenic acid is an omega-3 fatty acid because it has a double bond three carbons away from the "omega" carbon. By subtracting the highest double-bond locant in the scientific name from the number of carbons in the fatty acid we can obtain its classification. For arachidonic acid, we subtract 14 from 20 to obtain 6; therefore, it is an omega-6 fatty acid. This type of terminology is sometimes applied to oleic acid which is an omega-9 fatty acid.

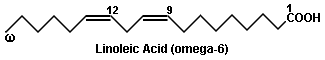
In these simplified structural formulas of unsaturated fatty acids, each angle represents a carbon atom. Notice that all the double bonds have the Cis configuration.
DHA (docosahexaenoic acid) and AA (arachidonic acid) are both crucial to the optimal development of the brain and eyes. The importance of DHA and AA in infant nutrition is well established, and both substances are routinely added to infant formulas. Excessive amounts of omega-6 polyunsaturated fatty acids and a very high omega-6/omega-3 ratio have been linked with pathogenesis of many diseases, including cardiovascular disease, cancer, and inflammatory and autoimmune diseases. The ratio of omega-6 to omega-3 in modern diets is approximately 15:1, whereas ratios of 2:1 to 4:1 have been associated with reduced mortality from cardiovascular disease, suppressed inflammation in patients with rheumatoid arthritis, and decreased risk of breast cancer. Some researchers have suggested that there is not very strong evidence for the benefits of these ratios, and that it may be better to increase the consumption of omega-3 fatty acids rather than decrease the consumption of omega-6 fatty acids because a reduction of polyunsaturated fats in the diet would increase the incidence of cardiovascular disease.