 Index
Index

Carbohydrates consist of the elements carbon (C), hydrogen (H) and oxygen (O) with a ratio of hydrogen twice that of carbon and oxygen. Carbohydrates include sugars, starches, cellulose and many other compounds found in living organisms. In their basic form, carbohydrates are simple sugars or monosaccharides. These simple sugars can combine with each other to form more complex carbohydrates. The combination of two simple sugars is a disaccharide. Carbohydrates consisting of two to ten simple sugars are called oligosaccharides, and those with a larger number are called polysaccharides.

Sugars are white crystalline carbohydrates that are soluble in water and generally have a sweet taste.
| Number of Carbons |
Category Name | Examples |
|---|---|---|
| 4 | Tetrose | Erythrose, Threose |
| 5 | Pentose | Arabinose, Ribose, Ribulose, Xylose, Xylulose, Lyxose |
| 6 | Hexose | Allose, Altrose, Fructose, Galactose, Glucose, Gulose, Idose, Mannose, Sorbose, Talose, Tagatose |
| 7 | Heptose | Sedoheptulose, Mannoheptulose |
Many saccharide structures differ only in the orientation of the hydroxyl groups (-OH). This slight structural difference makes a big difference in the biochemical properties, organoleptic properties (e.g., taste), and in the physical properties such as melting point and Specific Rotation (how polarized light is distorted). A chain-form monosaccharide that has a carbonyl group (C=O) on an end carbon forming an aldehyde group (-CHO) is classified as an aldose. When the carbonyl group is on an inner atom forming a ketone, it is classified as a ketose.

|

|
| D-Erythrose | D-Threose |
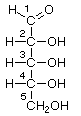 D-Ribose |
 D-Arabinose |
 D-Xylose |
 D-Lyxose |
The ring form of ribose is a component of ribonucleic acid (RNA). Deoxyribose, which is missing an oxygen at position 2, is a component of deoxyribonucleic acid (DNA). In nucleic acids, the hydroxyl group attached to carbon number 1 is replaced with nucleotide bases.

|

|
| Ribose | Deoxyribose |
Hexoses, such as the ones illustrated here, have the molecular formula C6H12O6. German chemist Emil Fischer (1852-1919) identified the stereoisomers for these aldohexoses in 1894. He received the 1902 Nobel Prize for chemistry for his work.
 D-Allose |
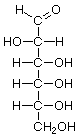 D-Altrose |
 D-Glucose |
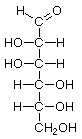 D-Mannose |
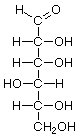 D-Gulose |
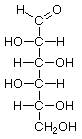 D-Idose |
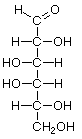 D-Galactose |
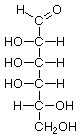 D-Talose |
Structures that have opposite configurations of a hydroxyl group at only one position, such as glucose and mannose, are called epimers. Glucose, also called dextrose, is the most widely distributed sugar in the plant and animal kingdoms and it is the sugar present in blood as "blood sugar". The chain form of glucose is a polyhydric aldehyde, meaning that it has multiple hydroxyl groups and an aldehyde group. Fructose, also called levulose or "fruit sugar", is shown here in the chain and ring forms. The relationship between the chain and the ring forms of the sugars is discussed below. Fructose and glucose are the main carbohydrate constituents of honey.
 D-Tagatose (a ketose) |
 D-Fructose |

 Galactose |
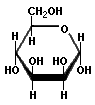 Mannose |

|

|
|
| D-Sedoheptulose | D-Mannoheptulose |
Many simple sugars can exist in a chain form or a ring form, as illustrated by the hexoses above. The ring form is favored in aqueous solutions, and the mechanism of ring formation is similar for most sugars. The glucose ring form is created when the oxygen on carbon number 5 links with the carbon comprising the carbonyl group (carbon number 1) and transfers its hydrogen to the carbonyl oxygen to create a hydroxyl group. The rearrangement produces alpha glucose when the hydroxyl group is on the opposite side of the -CH2OH group, or beta glucose when the hydroxyl group is on the same side as the -CH2OH group. Isomers, such as these, which differ only in their configuration about their carbonyl carbon atom are called anomers. The little D in the name derives from the fact that natural glucose is dextrorotary, i.e., it rotates polarized light to the right, but it now denotes a specific configuration. Monosaccharides forming a five-sided ring, like ribose, are called furanoses. Those forming six-sided rings, like glucose, are called pyranoses.
 D-Glucose (an aldose) |
 Cyclation of Glucose |
 α-D-Glucose |
 β-D-Glucose |
Saccharides with identical functional groups but with different spatial configurations have different chemical and biological properties. Stereochemisty is the study of the arrangement of atoms in three-dimensional space. Stereoisomers are compounds in which the atoms are linked in the same order but differ in their spatial arrangement. Compounds that are mirror images of each other but are not identical, comparable to left and right shoes, are called enantiomers. The following structures illustrate the difference between β-D-Glucose and β-L-Glucose. Identical molecules can be made to correspond to each other by flipping and rotating. However, enantiomers cannot be made to correspond to their mirror images by flipping and rotating. Glucose is sometimes illustrated as a "chair form" because it is a more accurate representation of the bond angles of the molecule. The "boat" form of glucose is unstable.
 β-D-Glucose |
 β-D-Glucose |
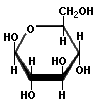 β-L-Glucose |
 β-L-Glucose |
 β-D-Glucose (chair form) |
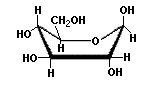 β-D-Glucose (boat form) |
Sugars may be modified by natural or laboratory processes into compounds that retain the basic configuration of saccharides, but have different functional groups. Sugar alcohols, also known as polyols, polyhydric alcohols, or polyalcohols, are the hydrogenated forms of the aldoses or ketoses. For example, glucitol, also known as sorbitol, has the same linear structure as the chain form of glucose, but the aldehyde (-CHO) group is replaced with a -CH2OH group. Other common sugar alcohols include the monosaccharides erythritol and xylitol and the disaccharides lactitol and maltitol. Sugar alcohols have about half the calories of sugars and are frequently used in low-calorie or "sugar-free" products.
Erythritol is a four-carbon polyol that is 60–70% as sweet as table sugar, but because it is only partially absorbed by the body it has only 0.2 Calories per gram, which is 95% less than table sugar. Erythritol is used as a food additive throughout much of the world. It is used in food for diabetics because it does not affect blood sugar and does not cause tooth decay. Although erythritol is well tolerated by humans, experiments show that erythritol is toxic to the fruit fly Drosophila melanogaster and may be useful as an insecticide.[5]
Xylitol, a five-carbon polyol which has the hydroxyl groups oriented like xylose, is a very common ingredient in "sugar-free" candies and gums because it is approximately as sweet as sucrose, but contains 40% less food energy. Although this sugar alcohol appears to be safe for humans, xylitol in relatively small doses can cause seizures, liver failure, and death in dogs.
Amino sugars or aminosaccharides replace a hydroxyl group with an
amino (-NH2)
group. Glucosamine is an amino sugar used to treat cartilage damage and reduce the pain and
progression of arthritis.
Uronic acids have a carboxyl group (-COOH) on the
carbon that is not part of the ring.
Their names retain the root of the monosaccharides, but the -ose sugar suffix is changed
to -uronic acid.
For example, galacturonic acid has the same configuration as galactose,
and the structure of glucuronic acid corresponds to glucose.


